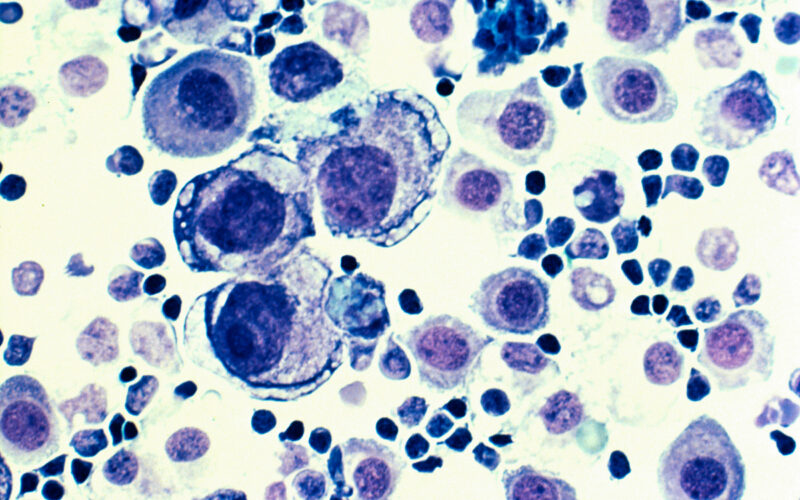
Recent advancements in cancer immunotherapy have brought promising news for patients battling head and neck squamous cell carcinoma (HNSCC). CEL-SCI Corporation’s Phase 3 clinical trial of Multikine® (Leukocyte Interleukin, Injection) has demonstrated significant improvements in overall survival and quality of life for individuals with locally advanced HNSCC. These findings, detailed in the peer-reviewed journal Pathology and Oncology Research, suggest a potential paradigm shift in the treatment of this challenging disease.
Understanding Head and Neck Squamous Cell Carcinoma (HNSCC):
HNSCC encompasses malignancies originating in the squamous cells lining the mucosal surfaces of the head and neck region, including the oral cavity, pharynx, and larynx. Globally, HNSCC is the seventh most common cancer, with approximately 890,000 new cases and 450,000 deaths annually. In the United States, these cancers account for about 4% of all cancer diagnoses, with an estimated 71,100 new cases projected in 2024.
Major risk factors for HNSCC include tobacco use, excessive alcohol consumption, and infection with high-risk human papillomavirus (HPV) types. The disease predominantly affects individuals over the age of 50, with a higher incidence observed in men compared to women.
The IT-MATTERS Study:
The Phase 3 clinical trial, known as the IT-MATTERS study, represents the largest endeavor of its kind in head and neck cancer, enrolling 928 treatment-naïve patients with resectable locally advanced primary squamous cell carcinoma of the oral cavity and soft palate. Participants were stratified into low-risk and high-risk groups based on their likelihood of recurrence post-surgery and were randomized to receive:
- Multikine combined with cyclophosphamide, indomethacin, and zinc (LI + CIZ) plus standard of care (SOC).
- Multikine plus SOC.
- SOC alone.
The Multikine regimen involved administering 400 International Units (IU) daily for five days a week over three consecutive weeks prior to surgery. Postoperative treatments included radiotherapy for low-risk patients and concurrent chemoradiotherapy for high-risk patients. The median follow-up duration was 56 months.
Key Findings:
- Overall Survival (OS): In the low-risk cohort treated with Multikine + CIZ + SOC, a significant OS advantage was observed. At 60 months, the OS rate was 62.7% for the Multikine group versus 48.6% for the SOC group, translating to a median OS benefit of 46.5 months (101.7 months vs. 55.2 months, respectively).
- Quality of Life (QoL): Assessments using the EORTC QLQ-C30 and EORTC QLQ-H&N35 questionnaires revealed that 95.1% of complete responders to Multikine reported improved QoL. Notably, complete responders experienced a 100% improvement on 60% of QoL measures, including reductions in head and neck pain, enhanced ability to eat, drink, and swallow, improved self-care capabilities, and better emotional well-being. Additionally, 89.4% of partial responders reported enhanced QoL metrics.
- Safety Profile: The incidence of treatment-emergent adverse events was comparable across all groups, with no additional safety concerns identified for Multikine over SOC alone post-surgery.
Implications and Future Directions:
The IT-MATTERS study suggests that neoadjuvant administration of Multikine, particularly when combined with CIZ and SOC, can significantly enhance overall survival and quality of life in patients with low-risk, locally advanced HNSCC. These promising results have led to the initiation of a final confirmatory Registration Study for Multikine in head and neck cancer, aiming to further validate its efficacy and safety.
Given the substantial global burden of HNSCC and the often debilitating effects of current treatment modalities, the introduction of Multikine as a neoadjuvant therapy could represent a significant advancement in the standard of care. Continued research and clinical trials will be essential to confirm these findings and potentially offer patients a more effective and tolerable treatment option.