It began with a recognition that many of the most important drivers of human disease were not easily addressed by traditional drug discovery approaches, particularly proteins that lacked well-defined binding pockets or enzymatic activity. For decades, these targets were considered undruggable, leaving entire disease pathways unexplored by modern medicine. As genomics, proteomics, and computational chemistry matured, a new opportunity emerged to go beyond inhibition and toward selective elimination of disease-causing proteins, opening a new frontier in precision medicine.
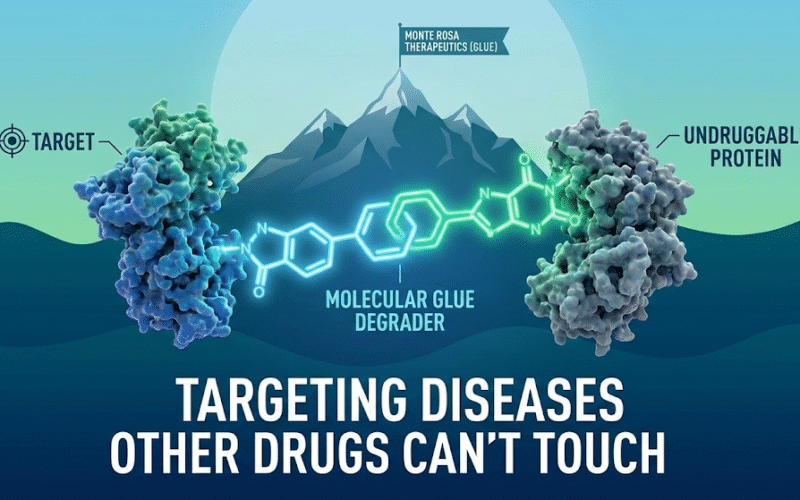
Monte Rosa Therapeutics (NASDAQ:GLUE) was founded to pursue that frontier through the development of molecular glue degraders, a novel class of small molecules that harness the cell’s natural protein disposal machinery to selectively remove pathogenic proteins. From its earliest days, Monte Rosa Therapeutics positioned itself not as a single-asset biotechnology company, but as a platform-driven organization designed to repeatedly discover and optimize new therapeutic candidates across multiple disease areas. The company built its scientific foundation around the idea that targeted protein degradation could unlock previously inaccessible biology, allowing researchers to directly modulate disease drivers at the protein level rather than simply blocking their activity.
Monte Rosa Therapeutics invested heavily in building a proprietary discovery engine known as QuEEN, which integrates computational modeling, artificial intelligence, structural biology, chemistry, and high-throughput proteomics. This platform enables Monte Rosa Therapeutics to identify and design molecular glue degraders with high selectivity and potency, while minimizing off-target effects. The QuEEN platform is not a single technology but an evolving system designed to generate a continuous pipeline of candidates, giving the company a repeatable innovation model rather than dependence on any single drug.
Over time, Monte Rosa Therapeutics expanded its focus beyond oncology into immunology, inflammation, cardiovascular disease, and other areas where protein dysregulation plays a central role. This strategic breadth reflects the company’s view that molecular glue degraders are not limited to one therapeutic category but represent a generalizable approach applicable across many diseases with high unmet medical need. By diversifying early, Monte Rosa Therapeutics positioned itself to participate in multiple large pharmaceutical markets while spreading clinical and regulatory risk.
The company’s growth also attracted the attention of major pharmaceutical partners, most notably Novartis, which entered into a broad strategic collaboration to leverage Monte Rosa Therapeutics’ degrader discovery platform for immune-mediated diseases. This partnership not only provided financial resources but also validated the scientific credibility of the platform at an industry level. Such collaborations are a key part of Monte Rosa Therapeutics’ business model, allowing it to retain core platform ownership while sharing development risk and accelerating commercialization through established global partners.
Monte Rosa Therapeutics has deliberately structured itself as a long-term science company rather than a short-term development vehicle. Its leadership has emphasized building internal capabilities in chemistry, biology, data science, and clinical development, enabling the company to advance programs from early discovery through human trials in-house. This integrated approach increases control over timelines, quality, and strategic direction, and supports the company’s ambition to become a sustainable, multi-product biotechnology enterprise.
As the biotechnology industry increasingly shifts toward precision medicine and individualized treatment strategies, Monte Rosa Therapeutics sits at the intersection of multiple transformative trends, including AI-driven drug discovery, targeted protein degradation, and platform-based R&D models. The company’s background reflects this convergence, blending cutting-edge science with scalable infrastructure to create a new generation of therapeutics that are both mechanistically precise and clinically impactful.
Today, Monte Rosa Therapeutics operates as a clinical-stage biotechnology company with a growing pipeline, a validated discovery platform, and a strategy centered on addressing fundamental biological drivers of disease. Its background is defined by its commitment to innovation, platform development, and long-term value creation, rather than reliance on any single asset or near-term commercial outcome. This foundation positions Monte Rosa Therapeutics as a company built not just to launch a drug, but to redefine how drugs are discovered and developed in the era of molecular precision.
A New Class of Cardiovascular and Inflammatory Medicines Is Emerging
Monte Rosa Therapeutics Inc. is positioning itself at the forefront of a new therapeutic paradigm by advancing molecular glue degrader technology into some of the most commercially significant and medically underserved disease areas. While the company initially drew attention for its oncology pipeline, recent data from its NEK7-directed molecular glue degrader program, MRT-8102, suggests that Monte Rosa may be on the verge of redefining how inflammatory and cardiometabolic diseases are treated, particularly atherosclerotic cardiovascular disease, chronic systemic inflammation, and NLRP3-driven disorders.
The NLRP3 inflammasome has emerged as one of the most important drivers of chronic inflammation in cardiovascular disease, metabolic disease, gout, pericarditis, and multiple autoimmune conditions. Traditional anti-inflammatory drugs operate upstream or downstream of this pathway and often carry limitations related to safety, efficacy, or immune suppression. Monte Rosa’s approach bypasses these limitations by targeting NEK7, a critical activator of the NLRP3 inflammasome, for selective degradation, rather than simple inhibition.
By eliminating NEK7 at the protein level, MRT-8102 disrupts inflammasome activation at its core, creating the potential for deeper, more durable, and safer inflammation control than current biologics or small-molecule inhibitors.

CHECK THIS OUT: Here’s Why Apogee Therapeutics (APGE) Is Suddenly on the Radar of Biotech Investors and Coeptis Therapeutics (COEP) Is Not Profitable Yet — and That’s Exactly Why It’s Interesting.
The Significance of an 85 Percent Reduction in CRP
The interim Phase 1 data from the GFORCE-1 study represents a major inflection point for Monte Rosa and for the broader field of cardiovascular inflammation. After just four weeks of dosing, MRT-8102 achieved a median 85 percent reduction in high-sensitivity C-reactive protein, one of the strongest biomarkers associated with cardiovascular risk, vascular inflammation, and future heart attack or stroke.
Even more compelling is the fact that 94 percent of subjects achieved hsCRP levels below 2 mg/L, a threshold widely associated with reduced cardiovascular disease risk and improved long-term outcomes. This level of biomarker suppression is comparable to, and in some cases exceeds, results seen with injectable biologics, yet MRT-8102 is orally bioavailable, offering substantial advantages in patient convenience, compliance, and healthcare system adoption.
This magnitude of effect positions MRT-8102 not as an incremental improvement, but as a potentially best-in-class therapy for inflammation-driven cardiovascular disease.
Deep and Sustained NEK7 Degradation Confirms the Mechanism
The clinical data confirms that Monte Rosa’s molecular glue degrader platform is functioning exactly as designed in humans. Across single ascending dose and multiple ascending dose cohorts, MRT-8102 demonstrated rapid, deep, and sustained degradation of NEK7, with approximately 80 to 90 percent target depletion observed in peripheral blood T cells.
This matters because biomarker effects without target engagement often fail to translate into durable clinical benefit. In this case, the pharmacodynamic data aligns tightly with the clinical biomarkers, confirming that NEK7 degradation directly drives reductions in CRP, IL-1β, and IL-6.
The consistency of NEK7 degradation across doses from 5 mg to 400 mg also suggests a broad therapeutic window and flexible dosing potential, which is particularly valuable for chronic diseases requiring long-term therapy.
Favorable Safety Supports Chronic Use
Perhaps just as important as the efficacy signal is the safety profile observed to date. MRT-8102 has demonstrated a favorable safety and tolerability profile with only mild to moderate adverse events, no dose-dependent toxicity, and no evidence of increased infection risk.
This is critical because systemic inflammation therapies often fail due to immunosuppression, infection risk, or long-term toxicity. The ability to safely suppress the NLRP3 pathway without impairing immune function would represent a significant breakthrough in chronic disease management.
The absence of increased infection risk strongly differentiates MRT-8102 from existing biologics targeting IL-1 or IL-6, which often carry black box warnings and high long-term safety monitoring burdens.
Expansion into Phase 2 Unlocks a Massive Commercial Market
Monte Rosa’s decision to expand GFORCE-1 and initiate the GFORCE-2 Phase 2 study in ASCVD in 2026 reflects strong internal confidence in the data and a recognition of the enormous commercial opportunity in cardiovascular disease.
ASCVD remains the leading cause of death globally and represents one of the largest pharmaceutical markets in the world. Even modest penetration into this space could represent multibillion-dollar revenue potential.
Moreover, the planned expansion into additional indications such as MASH, gout, and recurrent pericarditis further broadens the addressable market and increases the probability that MRT-8102 becomes a platform therapy rather than a single-indication drug.
Strategic Alignment With Big Pharma and Pipeline Depth
Monte Rosa’s collaboration with Novartis adds an additional layer of validation to its molecular glue degrader platform. Novartis’ plans to initiate Phase 2 studies of the VAV1-directed degrader MRT-6160 in immune-mediated diseases in 2026 further reinforce that the platform is scalable beyond NEK7 and beyond cardiovascular disease.
At the same time, Monte Rosa’s oncology pipeline, including MRT-2359 and future CDK2 and cyclin E1 degraders, ensures that the company maintains diversified exposure across multiple therapeutic areas.
This combination of cardiovascular, immunology, and oncology creates a rare balance between high-probability, high-market chronic disease programs and higher-risk, higher-reward oncology assets.
Why the Market May Be Underestimating GLUE Stock
Despite the strength of the clinical data and the breadth of the pipeline, Monte Rosa remains valued as a mid-stage biotech with modest expectations. That valuation does not reflect the potential of MRT-8102 to become a foundational therapy in cardiovascular disease, nor does it reflect the optionality embedded in the company’s molecular glue degrader platform.
If Phase 2 confirms the Phase 1 biomarker data in ASCVD patients, the narrative around GLUE stock could shift rapidly from speculative biotech to category-defining innovator.
The combination of deep biological validation, strong biomarker effects, favorable safety, oral dosing, and large unmet medical need creates an unusually attractive risk-reward profile for long-term investors.
Final Thought
Monte Rosa Therapeutics is no longer just an oncology-focused biotech. It is emerging as a platform company capable of redefining treatment paradigms across cardiovascular, inflammatory, autoimmune, and oncologic diseases.
MRT-8102’s early success represents not only a clinical breakthrough, but a strategic one, proving that molecular glue degraders can safely and effectively modulate complex human biology in chronic disease settings.
If this trajectory continues, Monte Rosa could become one of the most important biotechnology stories of the next decade.
That is why Monte Rosa Therapeutics Inc. and GLUE stock represent a compelling long-term bullish thesis grounded not in hype, but in mechanistic science, human data, and enormous unmet medical need.
READ ALSO: Could UnitedHealth Group (UNH) Be the Safest Way to Invest in Healthcare Growth? and SELLAS Life Sciences Group (SLS) Just Took a Big Step Toward Changing How Cancer Is Treated.