Founded in 2017 as a mission-driven spin-off from the University of Iowa, the Chicago-based healthcare technology firm was established to bridge the critical gap between laboratory breakthroughs in genomic science and the real-world clinical management of heart disease. Its scientific foundation is built upon a revolutionary understanding of the interplay between static genetic predispositions and the dynamic, reversible world of epigenetics, specifically focusing on how DNA methylation can act as a real-time indicator of cardiovascular health. The original vision was to eliminate the “guessing game” in heart disease prevention by developing the first-ever clinical blood tests that profile a patient’s unique molecular twin, a mission that has guided the organization through years of rigorous validation and the development of a proprietary artificial intelligence platform designed to interpret complex biological data.
Cardio Diagnostics Holdings Inc. (NASDAQ:CDIO) successfully established itself as a pioneer in the field of precision cardiovascular medicine by commercializing a unique dual-track approach that monitors both the “blueprint” of a patient’s DNA and the “activity” of their genes. Throughout its history, the company has prioritized the engineering of a scalable, AI-powered engine capable of processing massive datasets to identify coronary heart disease risk long before the onset of symptomatic episodes or the need for invasive diagnostic procedures. This persistent focus on structural innovation allowed Cardio Diagnostics Holdings Inc. to build a robust intellectual property portfolio that underpins its flagship products, Epi+Gen CHD™ and PrecisionCHD™, effectively moving the standard of care from reactive treatment to proactive, molecular-based prevention. By maintaining its commitment to rigorous clinical evidence, the organization has managed to preserve its role as a key disruptor in a global diagnostic sector that is increasingly shifting toward personalized, high-tech intervention.
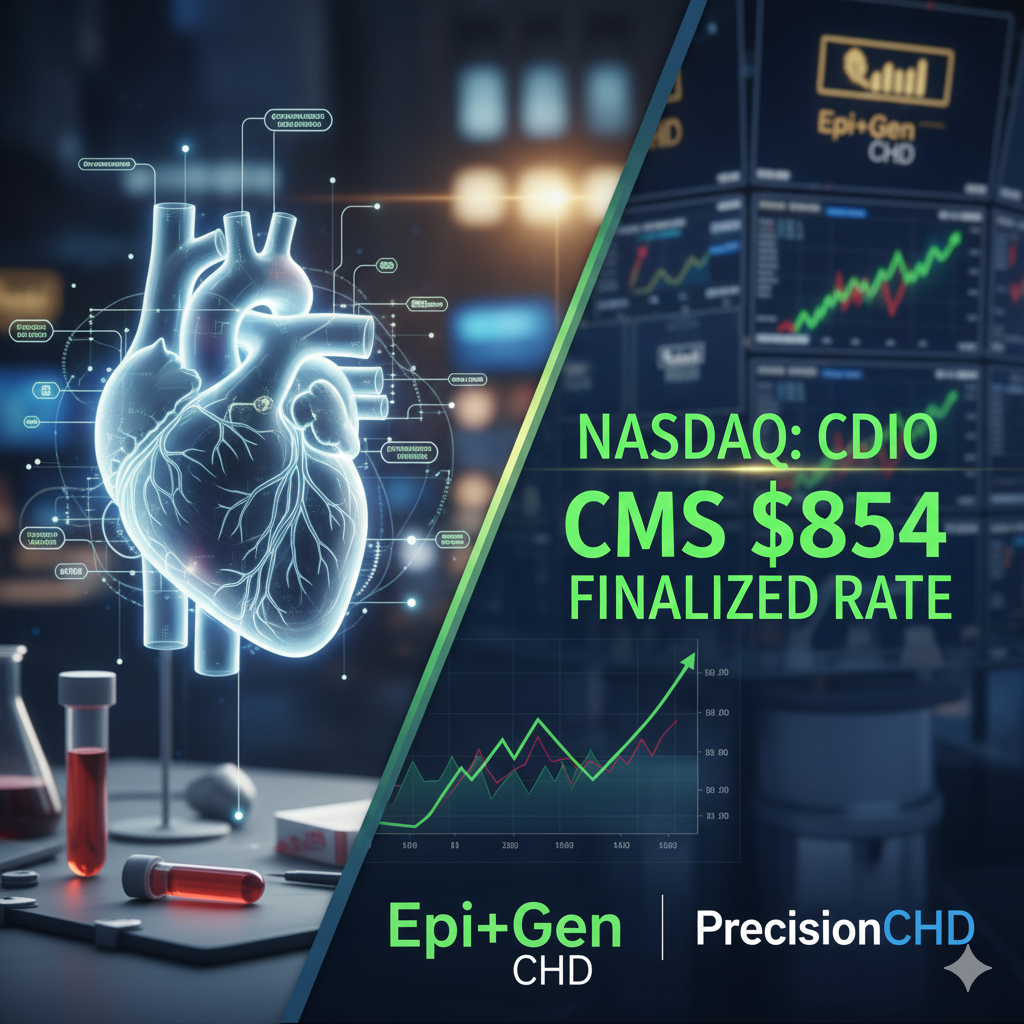
Cardio Diagnostics Holdings Inc. reached a transformative historical milestone in late 2025 by securing a finalized gapfill payment rate of $854 from the Centers for Medicare and Medicaid Services (CMS), marking its official transition from a research-intensive enterprise to a commercially viable leader in the healthcare market. This achievement was the culmination of years of collaborative research with academic institutions and private clinics, validating the company’s ability to detect “hidden” heart conditions such as INOCA and MINOCA which often escape traditional stress tests. By securing a high-value reimbursement pathway and expanding its reach through strategic provider networks, Cardio Diagnostics Holdings Inc. has positioned itself to address the primary cause of mortality in the United States on a massive, institutional scale. The company’s background is rooted in the belief that the future of cardiology lies at the intersection of genetic stability and epigenetic change, a philosophy that continues to drive its pursuit of a world where heart disease is managed as a chronic, predictable condition rather than an unpredictable emergency.
Cardio Diagnostics Holdings Inc. is also defined by its institutional resilience and its history of securing critical global patents, including a significant award in Japan that opened the door to international commercialization. These accomplishments are a testament to the company’s history of delivering high-quality, actionable data that assists clinicians in personalizing care for patients who have historically been underserved by standard diagnostic tools. Today, the organization stands as a cornerstone of the burgeoning AI-driven diagnostics ecosystem, leveraging specialized expertise in DNA methylation and bioinformatics to refine its cardiovascular risk-assessment models. As Cardio Diagnostics Holdings Inc. advances toward the full integration of its tests into primary care and specialty clinics, it remains committed to the patient-centric research and scientific transparency that have defined its corporate identity since its inception in the labs of the Midwest.
The $854 CMS Breakthrough: Cardio Diagnostics Holdings Inc. (NASDAQ: CDIO) Triggers a Massive Revenue Inflection Point
The landscape of cardiovascular medicine witnessed a seismic shift this December as Cardio Diagnostics Holdings Inc. (NASDAQ: CDIO) announced a monumental regulatory victory. The Centers for Medicare and Medicaid Services (CMS) has officially issued a final gapfill payment rate of $854 for both the Epi+Gen CHD™ and PrecisionCHD™ tests. This finalized rate represents a staggering increase from the preliminary figures of $350 and $684.76 respectively, signaling a powerful institutional validation of the company’s AI-driven epigenetic technology. Effective for claims starting January 1, 2026, this decision transforms Cardio Diagnostics from a speculative clinical innovator into a commercially primed powerhouse with a clear, high-margin reimbursement pathway for the nation’s most vulnerable patient population.
CHECK THIS OUT: Corcept (CORT) Skyrockets 1,534% in 10 Years and Immuneering (IMRX) Reports 86% 9-Month Survival in Pancreatic Cancer.
Redefining Precision Cardiology with Epigenetic and Genetic Integration
At the heart of the bullish thesis for Cardio Diagnostics Holdings Inc. is its unique ability to map the “molecular twin” of a patient’s cardiovascular health. While traditional heart tests often rely on static genetic markers or late-stage physiological changes, the Epi+Gen CHD™ platform integrates DNA methylation data with genetic variants to provide a real-time snapshot of heart disease risk. This dual-track approach allows physicians to see not just the “blueprint” of a patient’s heart, but the actual “activity” within those genes. By identifying coronary heart disease (CHD) at its earliest stages—often before a single symptom appears—the company is effectively providing a “early warning system” that could prevent a large percentage of the 370,000 annual deaths attributed to coronary complications in the United States.
Solving the Diagnostic Dilemma of “Hidden” Heart Disease
A significant portion of the medical community’s interest in Cardio Diagnostics centers on its ability to address Ischemia with No Obstructive Coronary Arteries (INOCA) and Myocardial Infarction with No Obstructive Coronary Arteries (MINOCA). Recent clinical data unveiled at the American Heart Association (AHA) Scientific Sessions demonstrated that PrecisionCHD™ can detect heart disease in patients who often receive “clear” results from traditional stress tests or angiograms. This is a critical breakthrough because nearly 40% of patients undergoing invasive procedures are found to have no obstructive disease despite remaining at high risk for a major cardiac event. By providing a non-invasive, blood-based diagnostic that carries the weight of a $854 Medicare reimbursement, Cardio Diagnostics is bridging a massive gap in the standard of care.
Strategic Commercial Expansion and Provider Network Acceleration
The $854 CMS final rate is the catalyst that the company’s commercial team has been preparing for throughout 2025. Over the last quarter, Cardio Diagnostics Holdings Inc. has aggressively expanded its provider network, adding fifteen new organizations across the country ranging from concierge health groups to primary care clinics. The company has also secured high-profile partnerships, such as its collaboration with AGEPHA Pharma to use PrecisionCHD™ as a companion diagnostic for LODOCO®, the first FDA-approved anti-inflammatory drug for cardiovascular disease. This “picks and shovels” model, where the company’s tests validate the efficacy of third-party pharmaceuticals, creates a secondary, high-growth revenue stream that complements its direct clinical sales.
Financial Turnaround and a Cleaned-Up Capital Structure
From an investment perspective, the structural changes at Cardio Diagnostics Holdings Inc. in 2025 have paved the way for a 2026 breakout. Following a 1-for-30 reverse stock split earlier in the year, the company regained Nasdaq compliance and consolidated its share count, creating a leaner vehicle for growth. With a current market capitalization that remains in the micro-cap territory, the firm is trading at a significant discount to its fundamental potential. When one considers that the company holds more cash than debt and has successfully slashed its quarterly burn rate, the $854 payment rate acts as a high-octane fuel for its valuation. Even a modest penetration into the Medicare market, which serves millions of Americans at high risk for heart disease, could result in a multi-bagger fundamental re-rating.
Global Patent Strength and International Commercialization
While the immediate focus remains on the lucrative U.S. Medicare market, the company has also fortified its global moat. In 2025, Cardio Diagnostics was awarded a comprehensive patent in Japan for its integrated genetic and epigenetic technology, opening the door to the third-largest healthcare market in the world. This international expansion strategy, combined with a growing portfolio of U.S. patents, ensures that the company’s AI-powered precision medicine platform remains protected from competitors. As the world moves toward more personalized, molecular-based diagnostic solutions, the “first-mover” advantage held by Cardio Diagnostics Holdings Inc. becomes increasingly valuable to potential M&A suitors in the big pharma and diagnostics space.
The Investor Narrative: A 2026 Breakout Target
The conclusion for investors is clear: the $854 CMS final rate is the “missing piece of the puzzle” that turns scientific excellence into a sustainable business model. With cardiovascular disease remaining the leading cause of death in America, the societal and economic demand for more accurate, accessible testing has never been higher. Cardio Diagnostics Holdings Inc. (NASDAQ: CDIO) is entering 2026 as one of the few micro-cap biotechs with a finalized, high-value reimbursement rate, a de-risked AI platform, and an expanding global footprint. For those looking for a high-conviction play in the precision medicine sector, the alignment of regulatory victory and commercial readiness makes CDIO a top candidate for institutional accumulation in the coming months.
READ ALSO: Tiziana (TLSA) Surges 143% in 2025 and Immuneering (IMRX) Reports 86% 9-Month Survival in Pancreatic Cancer.