We recently published our article Top 10 Cancer Biotech Small-Caps That Could Shock the Market Next. This piece looks at Oncolytics Biotech Inc. (NASDAQ:ONCY) as cancer biotech sentiment firms on renewed risk appetite and investors refocus on differentiated pipelines with meaningful upcoming catalysts that can change the company’s trajectory fast.
Cancer biotech has a funny habit: it can go quiet for months, then hijack the entire market in a single morning with one line of text—“met primary endpoint.” That’s not hype, that’s the business model. Oncology drug development is built around proof, and Wall Street prices proof with the emotional range of a weather app in a hurricane. One clean dataset can turn a forgotten small-cap biotech into the most talked-about ticker on NASDAQ or New York Stock Exchange, and one messy readout can erase a year of optimism before lunch. If you’ve been around the biotech tape long enough, you learn this simple rule: in cancer biotech, the calendar matters as much as the science. The real “earnings season” is often the stretch of Phase 2 results, Phase 3 topline data, and regulatory milestones that force investors to stop guessing and start pricing probabilities.
The Odd Trivia That Explains Why Small-Cap Oncology Stocks Move So Violently
Here’s a piece of trivia most people don’t think about until they’re staring at a biotech chart: cancer is not one disease, and “oncology” isn’t one market. It’s dozens of battlefields—solid tumors and hematologic malignancies, first-line and refractory settings, biomarker-defined subgroups, combination regimens, maintenance therapy, and the never-ending chess match of resistance. That complexity is exactly why oncology biotech stocks keep producing surprise winners. A therapy doesn’t need to cure everything; it needs to work clearly in the right slice of patients, with the right dosing, and the right safety profile. When that happens, the commercial runway can be real even if the initial indication is narrow, because success in one tumor type can open doors to label expansion, combinations, and adjacent indications.
Small-cap cancer biotech companies amplify this effect because their valuation is often concentrated in one or two core programs. That concentration is what investors mean when they talk about “asymmetric upside” in biotech stocks. A multi-billion-dollar pharma can absorb a failed trial. A small-cap oncology developer often can’t—and that’s precisely why it can shock the market next if the data lands the right way. The move isn’t just about revenue forecasts; it’s about probability rerating. The market goes from “maybe” to “measurable,” and the stock reprices like it’s waking up in a different universe.
Why “Clinical Catalysts” Are the Real Currency in Biotech Investing
In normal industries, investors obsess over sales cycles, margins, and guidance. In clinical-stage biotech, especially oncology, the language is different: primary endpoint, hazard ratio, durability of response, safety/tolerability, response rate, progression-free survival, overall survival, and whether the trial design matches what regulators and clinicians actually respect. That’s why biotech investors sound like part-time statisticians. A “major clinical catalyst” isn’t just news—it’s an evidence drop that changes the odds.
And yes, the U.S. Food and Drug Administration has a starring role in this drama. FDA approval, accelerated approval discussions, and PDUFA-style timelines are the moments when the market stops debating narrative and starts debating outcomes. If you’re trying to rank cancer biotech small-caps that could shock the market next, you’re really ranking schedules of truth: imminent trial readouts, regulatory decisions, and conference-driven data presentations that force the market to take a side.
The Not-So-Secret Reason These Names Can “Shock the Market”
Another trivia point that separates casual biotech watchers from seasoned ones: in oncology, “good enough” can be transformative. A drug doesn’t have to be perfect to win. It has to be meaningfully better than existing options in a setting where doctors are hungry for something that actually moves the needle. That’s why targeted therapy, precision oncology, antibody-drug conjugates, immunotherapy combinations, cell therapy, and next-generation oncology platforms keep producing these abrupt repricings. The bar isn’t “magic.” The bar is “clinically meaningful,” “statistically credible,” and “commercially adoptable.”
This is where small-cap biotech stocks get their torque. When a company shows a signal that looks reproducible—clean dose-response, consistent benefit across subgroups, manageable safety—investors don’t just model one indication. They start modeling the platform’s right to exist. And that’s when a market cap that looked tiny yesterday starts to look “wrong,” because the addressable opportunity suddenly feels larger and closer.
The Balance Sheet Angle Most People Miss Until It Matters
There’s a reason serious biotech investors keep one eye on the science and the other on the balance sheet. Oncology trials are expensive, timelines are unforgiving, and the market has zero mercy for companies that run out of cash right before a catalyst. So when we talk about “leverage” in small-cap cancer biotech, we’re not talking about debt-fueled financial engineering. We’re talking about runway. Cash runway. Optionality. The ability to reach the next readout without financing panic.
That’s why concepts like market cap, enterprise value, and net cash matter in this list. A company with a relatively low enterprise value versus market cap often has a bigger net-cash cushion, which can reduce near-term dilution risk and give management more flexibility to execute. It doesn’t guarantee success—nothing in cancer drug development does—but it changes the risk profile in a way that the market often rewards when catalysts approach.
What This Article Is Really About
This article isn’t pretending to predict the future with certainty—biotech doesn’t reward that kind of arrogance. What it does is map where the next shocks can come from in the cancer biotech landscape by focusing on small-cap oncology stocks with the kind of setups that historically create violent repricings: meaningful clinical catalysts, credible trial design, real shots on goal, and balance-sheet survivability. The companies on this watchlist sit in that sweet spot where expectations are still fragile enough to be surprised, but the catalyst calendar is loud enough to force a verdict.
If you’ve ever watched a biotech stock triple on a random Tuesday and wondered how anyone saw it coming, the answer is usually boring: somebody was tracking the timeline, the endpoints, the cash runway, and the odds—and they understood that in oncology, the market doesn’t move on vibes. It moves on data.
The Keywords Investors Keep Searching—Because They’re Actually the Rules of the Game
If you’re researching cancer biotech stocks to watch, oncology biotech small-caps, clinical-stage biotech companies, biotech catalysts, Phase 2 results, Phase 3 topline data, FDA approval risk, PDUFA timelines, immunotherapy stocks, targeted therapy, precision oncology, antibody-drug conjugates, CAR-T competitors, and biotech market cap rankings, you’re already speaking the language of how these stocks reprice. This introduction is built around the same reality: in 2026, the biggest market surprises often won’t come from the safest companies—they’ll come from the small-cap cancer biotech names where one dataset can flip the entire narrative.

CHECK THIS OUT: Why Crinetics Pharmaceuticals (CRNX) Is the “Slow Burn” Biotech Investors Love and Lexicon Pharmaceuticals (LXRX) Proves That Boring Science Can Still Move Markets.
Our Methodology
We screened U.S.-listed oncology and cancer-focused biotech stocks on the NYSE and NASDAQ and filtered for small-caps using current market capitalization, then narrowed the universe to companies with identifiable near-term value drivers such as upcoming clinical trial readouts, regulatory milestones, or meaningful program updates that could reprice expectations. We ranked the final ten from lowest to highest market cap for a clean, consistent size-based list, and we sanity-checked each pick using practical “shock potential” signals including enterprise value versus market cap (net-cash optionality), cash runway and dilution risk, liquidity/trading volume, pipeline concentration and trial stage, and the presence of clear catalyst timing rather than vague long-dated promises.
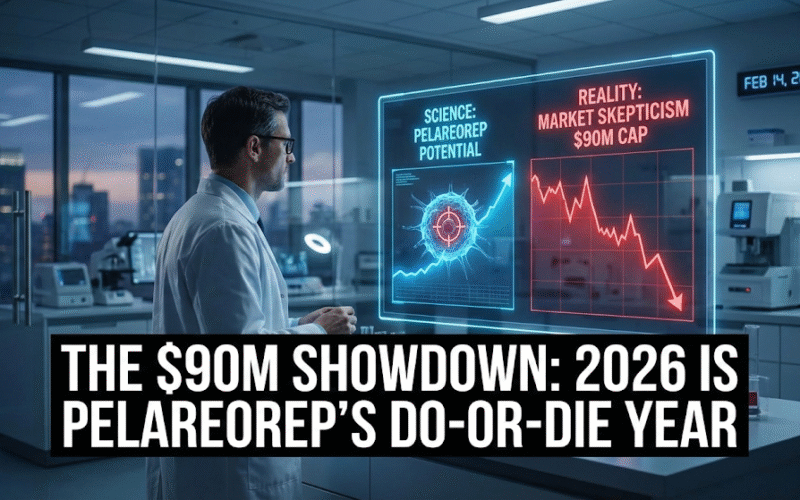
2. Oncolytics Biotech Inc. (NASDAQ:ONCY)
Market Cap: $90.12M
Leverage: 3.40%
Oncolytics Biotech is one of those clinical-stage oncology companies where the market often whipsaws between two extremes: “this is fascinating science” and “this will never translate.” The bullish thesis in 2026 is that the company is trying to move decisively out of the science-project bucket and into the “credible registration path” bucket by leaning on survival-oriented narratives in multiple tumor types, tightening its development focus, strengthening its intellectual property posture, and repeatedly signaling that it wants strategic partners to help drive late-stage execution. The asset at the center of everything is pelareorep, a systemically delivered oncolytic virus–based immunotherapy approach that the company positions as a way to activate anti-tumor immunity and potentially sensitize tumors to existing standards of care like chemotherapy and checkpoint inhibitors.
The reason this is investable, at least as a high-upside biotech stock idea, is that ONCY is not pitching pelareorep as a standalone “one drug, one tumor” product. It is pitching a combination-enabling platform that can add incremental benefit to established regimens in areas where outcomes remain poor and where the immunotherapy playbook still has gaps. In today’s oncology market, “incremental benefit in the right population” can be worth a lot, especially if the mechanism is plausible, the effect size is clinically meaningful, and the company can define a trial strategy that regulators and large pharma partners will take seriously.
If you’re researching immunotherapy stocks, oncolytic virus therapy, next-generation cancer immunotherapy, or undervalued oncology biotech names, Oncolytics is squarely in the high-torque category: small cap, pre-revenue, and therefore dependent on clinical proof and capital markets access, but with a narrative that can re-rate quickly if the company convinces investors that pelareorep is being advanced toward registration rather than perpetual early-phase exploration.
What Pelareorep Is and Why “Systemic Delivery” Is a Big Deal for Commercial Reality
Pelareorep is best understood as an immune-activating therapeutic built on oncolytic virus biology. Many oncolytic approaches in oncology have historically leaned on injecting directly into a tumor lesion. That can work in accessible tumors, but it creates obvious limitations for metastatic disease and broad adoption. Oncolytics emphasizes systemic intravenous delivery, which is strategically important because it supports the company’s core claim: pelareorep can be used across multiple advanced cancer settings and combined with standard therapies in a way that is scalable for real-world oncology practice.
For investors, systemic delivery increases the addressable opportunity if the data cooperate. It makes it easier to imagine a future where pelareorep becomes a standard add-on in defined regimens, rather than an “academic center only” procedure-dependent therapy. This is also exactly how modern oncology adoption happens: a therapy wins when it fits into existing treatment flow, not when it forces the whole system to change.
From an SEO lens, this is why the Oncolytics story lines up with high-intent keywords: systemic oncolytic virus, IV immunotherapy, combination immunotherapy, tumor microenvironment activation, and turning “cold tumors” into “hot tumors.” Those phrases are not just marketing buzz. They describe the clinical problem that keeps showing up across solid tumors: many cancers do not generate enough immune engagement for checkpoint inhibitors to work well, and the industry is still searching for reliable “priming” solutions.
Breast Cancer as the Credibility Anchor: Why BRACELET-1 Matters to the Bull Case
In oncology biotech investing, randomized Phase 2 data are often the dividing line between “interesting concept” and “credible signal.” Oncolytics has leaned on BRACELET-1, a randomized Phase 2 study in HR-positive, HER2-negative metastatic breast cancer evaluating paclitaxel alone versus regimens that add pelareorep (and, in some arms, additional immunotherapy components). The company’s messaging around this dataset has emphasized meaningful improvements in progression outcomes when pelareorep is combined with standard therapy, which is the kind of claim that forces investors to pay attention—because HR+/HER2– metastatic breast cancer is a competitive area where weak signals get ignored.
The bullish interpretation is not “Phase 2 equals approval.” The bullish interpretation is that the magnitude and direction of the benefit being discussed is the type of evidence that can justify a registration-enabling plan if it holds up under stricter trial design. Breast cancer is also strategically valuable because it is a field where clinicians care deeply about progression and durability, and where combination strategies must show a clear value-add to justify use.
What investors want to see from Oncolytics in 2026 is continued clarity on how BRACELET-1 data translate into a concrete late-stage roadmap. If the company can define the right patient selection logic, the right combination backbone, and the right endpoints, breast cancer can become the clinical foundation that supports broader platform value.
Pancreatic Cancer: Why Survival Signals Here Can Change a Company’s Fate
Metastatic pancreatic ductal adenocarcinoma is one of the harshest proving grounds in oncology. That’s why any credible survival improvement narrative in this disease can reprice a clinical-stage company fast. Oncolytics has repeatedly highlighted survival-oriented data themes in pancreatic cancer settings involving pelareorep combined with chemotherapy. Even if investors remain cautious about study size, design, and comparability, the direction matters because pancreatic cancer is a place where “small improvements” still matter clinically and commercially.
The bullish thesis here is strategic rather than purely statistical. If pelareorep is doing what it is supposed to do—creating immune activation and altering the tumor microenvironment—then pairing it with chemotherapy in pancreatic cancer could, in theory, push outcomes beyond what chemo alone can reliably deliver. If Oncolytics can build a credible trial plan that isolates pelareorep’s contribution and demonstrates reproducibility, pancreatic cancer becomes not just an indication but a partnership magnet. Big pharma partners pay attention to pancreatic cancer because the unmet need is enormous and the commercial opportunity is meaningful when a therapy can genuinely differentiate.
The market doesn’t need Oncolytics to already have a Phase 3 win in pancreatic cancer to start revaluing ONCY. It needs a believable plan to get there, and enough data to make that plan feel rational.
Colorectal Cancer and KRAS-Mutant Strategy: “Right Biology, Right Subset” as a De-Risking Move
One of the most practical ways to improve the probability of success in clinical oncology is to narrow the target. Instead of trying pelareorep broadly in every population, the company has emphasized specific data themes in difficult subsets, including KRAS-mutant metastatic colorectal cancer. KRAS-mutant tumors are often resistant and biologically challenging, which makes them useful “stress tests” for whether an immune-activating approach is truly doing something meaningful.
From an investor perspective, the value here is coherence. If Oncolytics can tie pelareorep’s mechanism to tangible changes in tumor biology and pair that with clinical signals in resistant subsets, the story becomes easier to underwrite. It also makes it easier for the company to design future trials with smarter selection criteria, which is exactly what regulators and partners want: a therapy used where it is most likely to deliver clear benefit.
From an SEO perspective, this aligns with strong search intent around KRAS-mutant cancer therapy, colorectal cancer immunotherapy combinations, and strategies to sensitize tumors to checkpoint inhibition. The more Oncolytics can attach pelareorep to specific, defensible biology-driven use cases, the more investable the platform looks.
Corporate Posture in 2026: Partners, Structure, Insider Signals, and a “More Serious” Development Tone
Small biotechs often talk about partnerships indefinitely. Markets only start believing it when the company’s behavior suggests it is setting itself up for larger-scale execution. Oncolytics has repeatedly communicated a desire to pursue strategic partnerships to accelerate development and maximize commercial impact. That’s not a guarantee that a deal will happen, but it’s a consistent theme and it matters because pelareorep’s best path to commercialization may involve shared development costs, bigger trial infrastructure, and partner validation of the mechanism.
The company has also discussed corporate structuring steps aligned with U.S. capital markets preferences, which can matter for investor access and friction reduction even if it doesn’t change the science. Another sentiment factor that tends to draw attention in micro-cap biotech is insider buying behavior; ONCY has seen at least one notable open-market insider purchase disclosed in early 2026, which doesn’t prove success but does add a small credibility signal that leadership believes risk/reward is attractive at the time of purchase.
These “corporate tells” don’t replace clinical data. But when combined with credible data direction, they can improve the market’s willingness to believe that ONCY is aiming for a real late-stage path rather than staying in the perpetual trial loop.
Intellectual Property as a Value Multiplier: Why Manufacturing and Method-of-Use Patents Matter
In platform-like therapeutics, investors often focus entirely on efficacy and ignore intellectual property until it becomes the headline. Oncolytics has been publicly emphasizing a 2026 intellectual property strategy that includes manufacturing-related patent filings and method-of-use protections aimed at extending the durability of commercial value if pelareorep reaches the market. It has also pointed to an accelerated patent examination pathway timeline for at least one filing, which could create an IP milestone within 2026.
This matters because if pelareorep becomes clinically important, manufacturing consistency and process know-how become part of the moat. Process IP can also be partner-friendly: it strengthens the economics of long-duration commercialization by reducing perceived vulnerability. In biotech valuations, a credible IP runway can materially increase probability-weighted value because it impacts how long a product can generate protected revenue.
The Real Risk: Financing and the “Micro-Cap Biotech Tax”
Oncolytics remains a clinical-stage company with no product revenue. That means funding risk is real. In biotech, “great science” can still be a bad stock if financing is punitive and recurring. The bearish scenario is not hard to imagine: trials take longer than expected, markets tighten, and the company raises money at low prices, forcing dilution that overwhelms per-share upside.
The bullish thesis doesn’t deny this. It argues that the best way to neutralize financing risk is to increase the credibility of a registration path and to land a partnership that changes the funding equation. If Oncolytics can convince the market that pelareorep is being advanced toward registrational studies in specific high-value settings—and if it can translate that into partner interest—then financing becomes less destructive and more strategic.
This is why 2026 is an important period. ONCY’s upside depends on it shifting from “interesting data” to “registrational intent,” and then to “registrational execution.”
What Would Make ONCY a Breakout Oncology Stock in 2026–2027
ONCY becomes a breakout stock when three conditions line up.
The first is clinical coherence. Pelareorep needs to keep producing meaningful benefit signals in repeatable settings, and the company needs to explain why those settings make sense biologically. Investors don’t need the therapy to work everywhere. They need it to work clearly somewhere.
The second is a believable registration roadmap. The company must show it has a practical path toward trials that regulators would recognize as approval-enabling, with defined endpoints and a design that can withstand scrutiny. “Registration-enabling” can’t just be a phrase; it has to look like an executable plan.
The third is capital alignment. Either the company maintains a runway that allows it to run the right trials without panic, or it lands partnerships that reduce the need for destructive financings. If those three conditions converge, the market can re-rate ONCY as a platform immunotherapy asset rather than a perpetual micro-cap experiment.
Bottom Line: Why ONCY Can Be a High-Upside Immunotherapy Bet Right Now
Oncolytics Biotech is best framed as a high-beta, high-upside immuno-oncology platform bet built around pelareorep, a systemically delivered oncolytic virus approach designed to activate anti-tumor immunity and improve outcomes when combined with established therapies. The company’s bullish narrative rests on the idea that it has moved beyond “intriguing biology” into a phase where randomized data in breast cancer and survival-oriented themes in hard tumors like pancreatic cancer justify serious registration planning. At the same time, Oncolytics is trying to strengthen the surrounding value drivers that sophisticated investors care about: partnership posture, U.S.-market alignment, and intellectual property durability.
If pelareorep’s signals remain consistent and the company translates them into a tighter late-stage roadmap—especially with external partner validation—ONCY can re-rate sharply because the market will start paying for probability-weighted commercialization instead of discounting the company as an endless early-phase story. If it fails to translate signals into credible late-stage intent, then ONCY remains exposed to the classic micro-cap biotech trap: promising science, slow timelines, and financing dilution.
READ ALSO: Here’s Why Apogee Therapeutics (APGE) Is Suddenly on the Radar of Biotech Investors and Coeptis Therapeutics (COEP) Is Not Profitable Yet — and That’s Exactly Why It’s Interesting.
Disclosure: No relevant interests to disclose. This article was originally published on BioTech HealthX.